On This Page
Background to: An electrochemical quartz crystal microbalance study of poly(acrylonitrile) deposition initiated by electrogenerated superoxide
On This Page
Electrodeposition of polymers
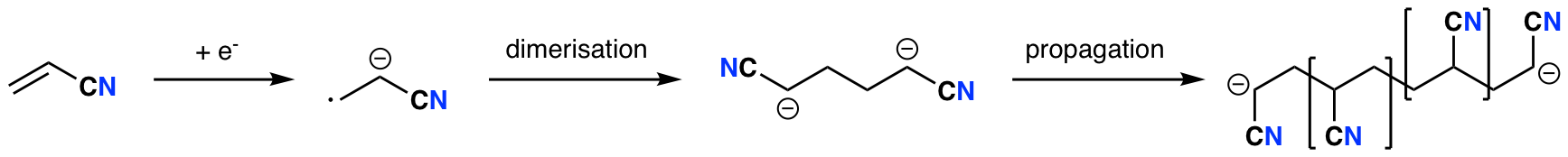
My PhD work was focused on the electrodeposition of polymers for use as electrolytes in so-called 3D batteries. When I started my PhD in 2008, I started out following the work of a previous postdoc, Gaber El-Enany, who had been studying the electrodeposition of poly(acrylonitrile) (PAN). The principle was quite straightforward: acrylonitrile can accept an electron to give a radical anion, which quickly dimerises; in aprotic solvents, the resulting dianion polymerises. (Trivia: in a protic solvent, the dianion protonates immediately to give adiponitrile - this is used industrially to produce 1,6-diaminohexane, a major precursor for nylon.) If the solvent is a poor solvent for the polymer (and many are), then the polymer precipitates out on the electrode surface as a film. We can then make the polymer into a Li+-conducting electrolyte by swelling it with a liquid electrolyte to make a gel.
In these early experiments I was using solutions of acrylonitrile in acetonitrile, with tetrabutylammonium perchlorate as a supporting electrolyte. This worked fine, we could make electrolyte films this way: this was eventually the topic of my first publication in 2009.
However, extremely negative potentials (i.e., very reducing conditions) were required to reduce acrylonitrile to the radical anion, and under those conditions the polymer was prone to partly undergo a further polymerisation reaction involving the nitrile side groups to form a “ladder” polymer:

This was easily apparent from Raman spectroscopy and the fact that the films were yellow when they should have been white!
Most of the early experiments were also run without first purging the oxygen out of the solution. The oxygen/superoxide redox couple was apparent in electrochemical experiments, but purging with argon gas did not have a noticeable effect on the polymerisation reaction. It did, however, have a noticeable effect when I started to change the concentration of acrylonitrile in the electrolyte.
Oxygen electrochemistry in acrylonitrile
In pure acrylonitrile (with only supporting electrolyte), the electrochemistry was very different: in cyclic voltammetry, only the oxygen reduction peak was seen, with the current rapidly dropping to zero afterwards and staying that way (the CV is shown below, reproduced here with permission).
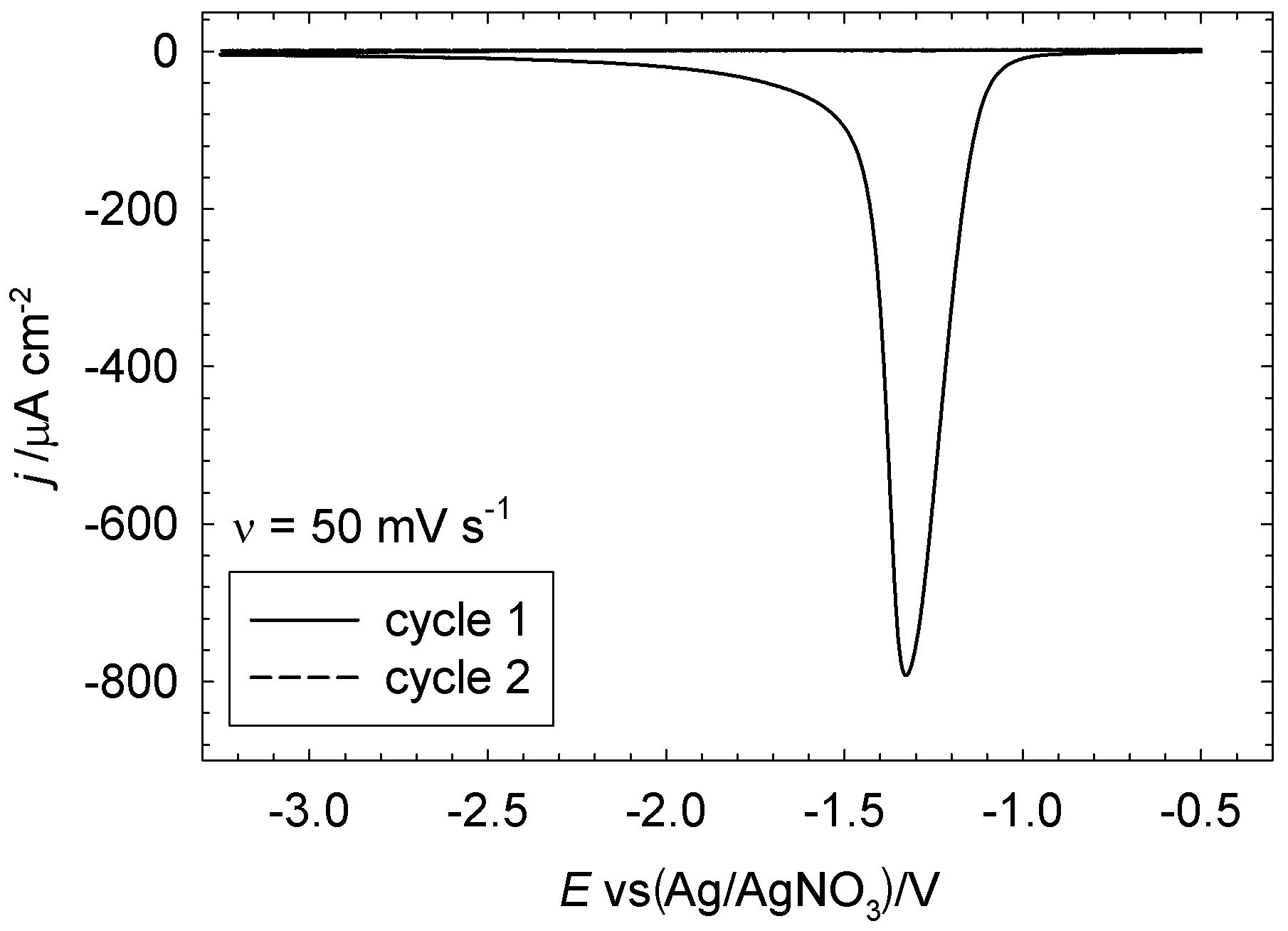
More surprisingly, the film on the glassy carbon electrode surface was perfectly white, and, according to Raman spectroscopy, was poly(acrylonitrile) with none of the ladder polymer impurities we saw before. Apparently, the nucleophilic superoxide formed from the reduction of dissolved oxygen initiated the polymerisation of acrylonitrile, but at a much more positive potential than the direct reduction of the monomer - avoiding the formation of the ladder polymer. This was interesting, because there did not seem to be any mention of a polymerisation reaction initiated by electrogenerated superoxide in the literature, though the use of potassium superoxide as an initiator for a conventional polymerisation reaction had previously been reported. (These first experiments were also done in 2009, at which time the reactivity of superoxide towards organic solvents in the context of the Li-air battery was not that widely realised.)
EQCM
I didn’t do much with the above results at the time - mostly because my PhD project called for moving to studying polyether-based systems instead. Also, although the idea of a more pure polymer produced under less harsh conditions was appealing, the polymers were a bit more difficult to swell with liquid electrolyte which made electrolyte tests a little more difficult anyway.
I however came back to this months later when I started using the Electrochemical Quartz Crystal Microbalance (EQCM), and I was interested in studying this process as one of a few different electropolymerisation reactions I was looking at at the time.
EQCM is a wonderfully powerful tool for studying electrodeposition, and works by measuring the resonant frequency of a precisely-cut quartz crystal which has very thin electrode films (usually of gold or platinum) deposited onto its surface. Under certain conditions, the frequency change can be directly correlated with the mass of the deposited film, and simultaneously recording the electrochemical response of the electrode and mass changes at the electrode gives an enormous amount of information about the deposition process.
In the case of the polymerisation discussed here, EQCM showed very clearly that there was a sudden increase in mass on the (gold) electrode surface taking place at the same time as the reduction of oxygen - clear evidence that superoxide initiates the polymerisation of acrylonitrile. Most usefully, correlating the mass change (assuming the Sauerbrey equation holds) lets us calculate the Faradaic efficiency for the reaction (i.e., how many moles of acrylonitrile monomers are deposited per mole of charge). For the first two CV cycles at 50 mV s-1, more than 70 acrylonitrile molecules were deposited for every electron transferred (one electron reduces superoxide and initiates the polymerisation; the propagation of the polymer chain is then only a chemical reaction).
This makes this process a rather efficient electrodeposition process - especially compared to the polyethers I later investigated.
Although these experiments were conducted around 2010, we ended up sitting on these results for a long time and only published in early 2013, after I had completed my PhD and moved to Sweden. Better late than never! It has hardly been cited since, however, but it’s still an interesting electropolymerisation process that hadn’t previously been reported.
comments powered by Disqus