On This Page
Background to: A redox shuttle to facilitate oxygen reduction in the lithium-air battery
On This Page
LABOHR
I started working within the FP7 project “LABOHR” (Lithium-Air Battery with split Oxygen Harvesting and Redox processes) full-time as a postdoc in March 2012. The aim of the LABOHR project was to demonstrate a new type of lithium-air (Li-air, or lithium-oxygen, Li-O2) battery design which somewhat resembles a flow battery: the oxygen would be harvested somehow from the air and forced into the electrolyte in a separate device or compartment, and pumped around to the cell itself where it would react at the positive electrode, and discharge the battery. The point of this was that it would significantly speed up the transport of oxygen to the electrode, compared to the more conventional “static” metal air cell design, and allow for higher rates of discharge as a result. One of the (many!) limitations of the system from a electric vehicle perspective in the conventional setup is the low solubility of oxygen in most electrolytes.
It’s an interesting approach, though the basic idea only tackles one of several mass transport limitations in the Li-O2 system. In principle, solid lithium peroxide (Li2O2) is still formed at the positive electrode as the discharge product, which can passivate (clog up) the electrode, and then you have the problem of even slower transport of the solid (and essentially insoluble) discharge product to the electrode surface when you want to recharge the battery again.
This is where the concept of a redox shuttle, or more accurately redox mediator, comes in.
Redox mediation
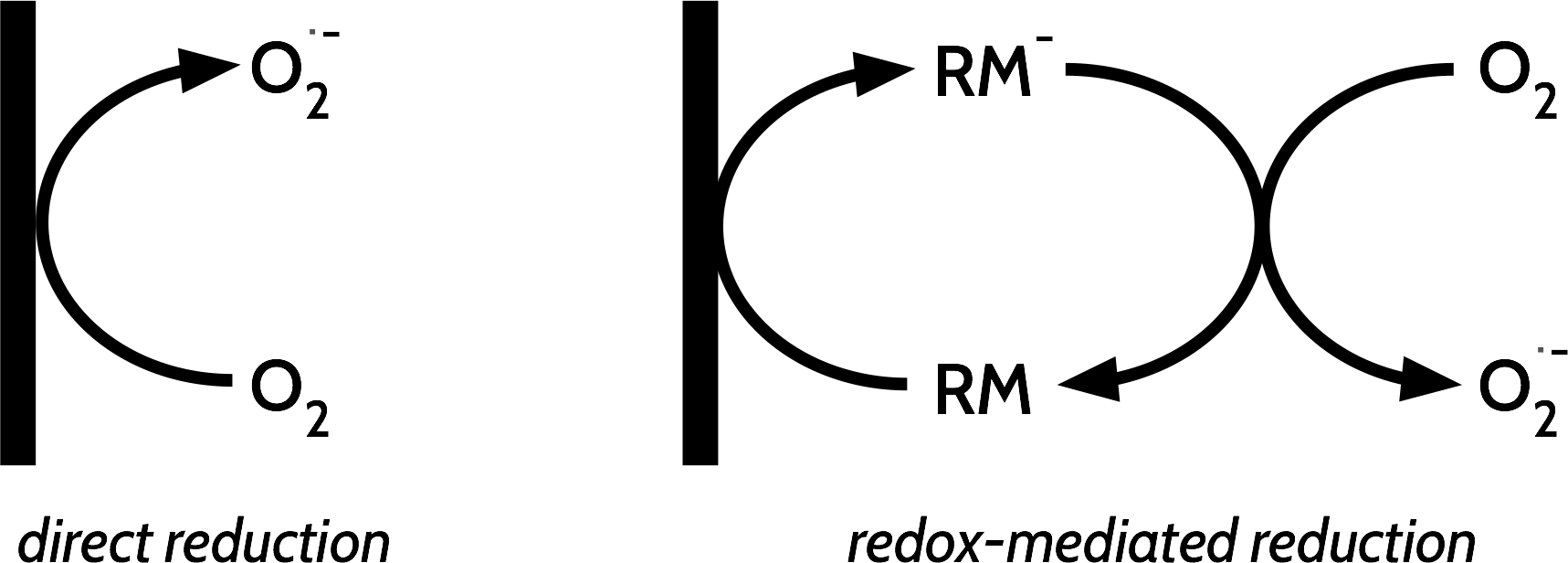
A redox mediator is another compound added to the electrolyte which is used to transfer electrons between the electrode surface and whatever substance it is we actually want to reduce or oxidise (in this case, oxygen, which undergoes a one-electron reduction to form superoxide, O2.-, which then disproportionates into Li2O2). A redox mediated electron transfer process can be more efficient than the direct electron transfer between the electrode and the substance if the direct electron transfer is slower because of kinetic or transport limitations.
In the case of the LABOHR cell design, there was another particularly good reason to look at a redox mediated cell reaction. If we could identify a redox mediator which was stable and highly soluble in the electrolyte, we could instead pump the redox mediator around the system and let it mediate the reduction of oxygen at the air/electrolyte interface, instead of pumping dissolved oxygen around the cell to reduce it at the electrode/electrolyte interface. In this case, the Li2O2 discharge product would form somewhere far away from the electrode surface, and therefore it wouldn’t be able to clog the electrode up - then we’d be able to discharge for longer and in turn increase the energy density of the system. Then, all we’d need is a second mediator for the oxidation of Li2O2 to reverse the process.
My supervisor at the time, Prof John Owen, had floated the idea of this approach early in 2012, and thought cobaltocene, with a standard potential a little over 2 V vs Li/Li+, might do the job. However, the tendency of cobaltocene to react with oxygen and immediately form covalent adducts with oxygen (not a good thing) seemed to be well-established. I instead suggested viologens - 4,4’-bipyridinium salts which are well known to undergo one-electron reductions to form radical cations which can then very readily reduce oxygen. Paraquat is a viologen which is widely used as a herbicide - in fact it works by interfering with electron transfer processes in biological systems, producing superoxide which causes cell death - making viologens rather toxic to plants and animals alike. (This is not a particular downside to viologens, you could expect that any redox mediator capable of mediating the same reaction would be toxic for the same reason.)
The viologen we used - I’d chosen ethyl viologen, mostly because of its availability - is very soluble, at least two orders of magnitude more soluble in the ionic liquid electrolyte we were using than oxygen under standard conditions.
The experiment
To test this, I had the glass cell shown in the photo below constructed. The cell has two compartments separated by a glass frit, which prevents the solutions in each compartment from mixing. The positive electrode compartment (on the left-hand side) contains the electrolyte solution (ionic liquid with a very small amount of lithium salt) with a low concentration of dissolved viologen, and two electrodes: one a large piece of platinum gauze, and the other a glassy carbon disk. The other compartment contains the electrolyte without any viologen, and the negative (Li metal) electrode.
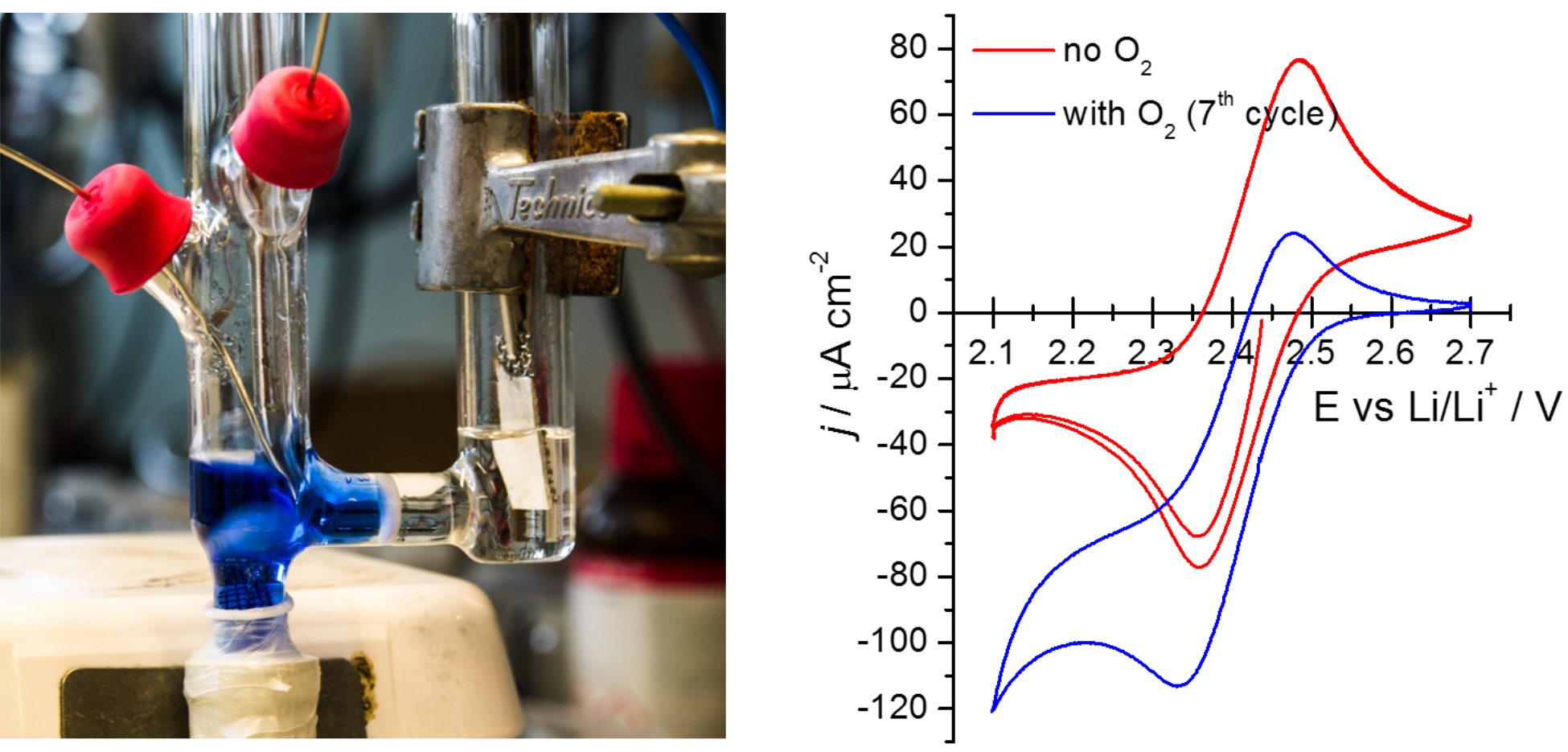
The experiments were relatively straightforward: I would first electrolyse (reduce) half of the viologen in the positive electrode compartment so that the concentrations of the oxidised and reduced forms of viologen are equal. The initial oxidised form of viologen, EtV2+, is colourless, and the reduced form, EtV·+, has the lovely deep blue colour you can see above. In an oxygen-free atmosphere, the viologen shows nicely reversible behaviour, as evidenced by cyclic voltammetry (CV, above right). I then continued the CV experiment while slowly bubbling oxygen gas into the positive electrode compartment. When the oxygen is added, the blue colour disappears entirely within a couple of minutes, and the CV curve shifts downwards.
This behaviour is exactly what we expect from a redox-mediated reaction such as this: the very fast reaction of EtV·+ with oxygen regenerates EtV2+, which enhances the reduction peak (negative current) and suppresses the re-oxidation peak (positive current). (In electrochemistry, we call this an EC’ reaction mechanism.)
The question now is whether the redox-mediated reduction actually generates the Li2O2 discharge product we expect? Unfortunately, doing repeated cycles of generating EtV·+ and bubbling with oxygen results in obvious decomposition (the electrolyte goes brown). In fact, the ability of superoxide to react with and degrade EtV2+ is already known. And really, this was not surprising - the ability of superoxide and other reduced oxygen species to react with almost all organic compounds is very well known. However, in the presence of a large amount of lithium salt, the disproportionation of superoxide is accelerated, in which case we can do more repeated electrolysis/O2 bubbling cycles before we see decomposition and we can electrochemically re-oxidise something which is consistent with Li2O2. At the time of publishing, we didn’t have direct evidence for Li2O2 formation, but this was confirmed in later work (along with a previously unknown ability of EtV·+ to also speed up the disproportionation of superoxide).
In context
A similar concept for the mediated oxidation of Li2O2 by the I-/I2 couple (to address the slow transport of the solid discharge product) was presented by Liox Power at the International Meeting on Lithium Batteries (IMLB) in Korea in June 2012, while we were working on the viologen experiments. Around the same time I was also doing experiments with the iodide salt of ethyl viologen (which was commercially available), which showed the behaviour of both mediated reactions in a single compound - however, this didn’t make it into this publication. I might have continued along that line but shortly after the IMLB meeting I agreed to move to Uppsala, so for that reason as much as anything else this needed to be wrapped up.
Our work was accepted by Electrochemistry Communications in October 2012, as far as I know the first report of a redox-mediated oxygen reduction reaction (ORR) in the Li-O2 system and the first report of an organic redox mediator for the system. Peter Bruce’s group published their work on an organic mediator (TTF) for the charge reaction a few months later, and the topic has been a rather active line of research in the field since then. To my disappointment a recent review on redox mediators for the Li-O2 system gave Peter Bruce’s group the credit for the first report of an organic mediator in this system and didn’t mention our paper (or the ones subsequently published by the group after I left). There’s no doubt that Peter’s group has done some of the best work in this area in recent years but I hope that others in the field don’t forget about the smaller players. At the time the idea of using a redox mediator in this system was not obvious, and it seems like it took a few years before many others started to consider the idea of a mediated ORR important (the appeal for the oxygen evolution reaction, OER, was more apparent). We also didn’t have some of the fancier techniques available to other groups doing Li-air research at the time, but almost six years on I’m still happy with this paper.