Are Prussian Blue analogue (PBA)-based Na-ion batteries safe?
Are Prussian Blue analogue (PBA)-based Na-ion batteries safe? Safer than LFP? It’s been argued to me a number of times that no oxygen in the cathode means no thermal runaway, but is that true? I think the evidence suggests no, and the hazards may actually be quite significant:
For those not familiar with Na PBAs, they are compounds with general formula NaxM[M’(CN)6] where M and M’ are transition metals (e.g. Fe, Ni, Mn). The structures are quite stable but in principle can decompose at high temperatures, releasing the cyanide (CN) ligand.
Also - worth noting that CN accounts for ~50 wt% of the cathode, so it is present in pretty large amounts.
This presents a few possibilities: generation of hydrogen cyanide gas (HCN, very toxic), cyanogen ((CN)2, rocket propellant, also very toxic), and the cyanide anion (CN-) itself, which is a good nucleophile (i.e., reactive). Is there any evidence for this?
Actually, some: a paper from my old group looked at the thermal stability of ‘Prussian White’ (M & M’ = Fe) and found that when fully charged, at ~300 °C there was a ~15% mass loss dominated by evolution of HCN and (CN)2.
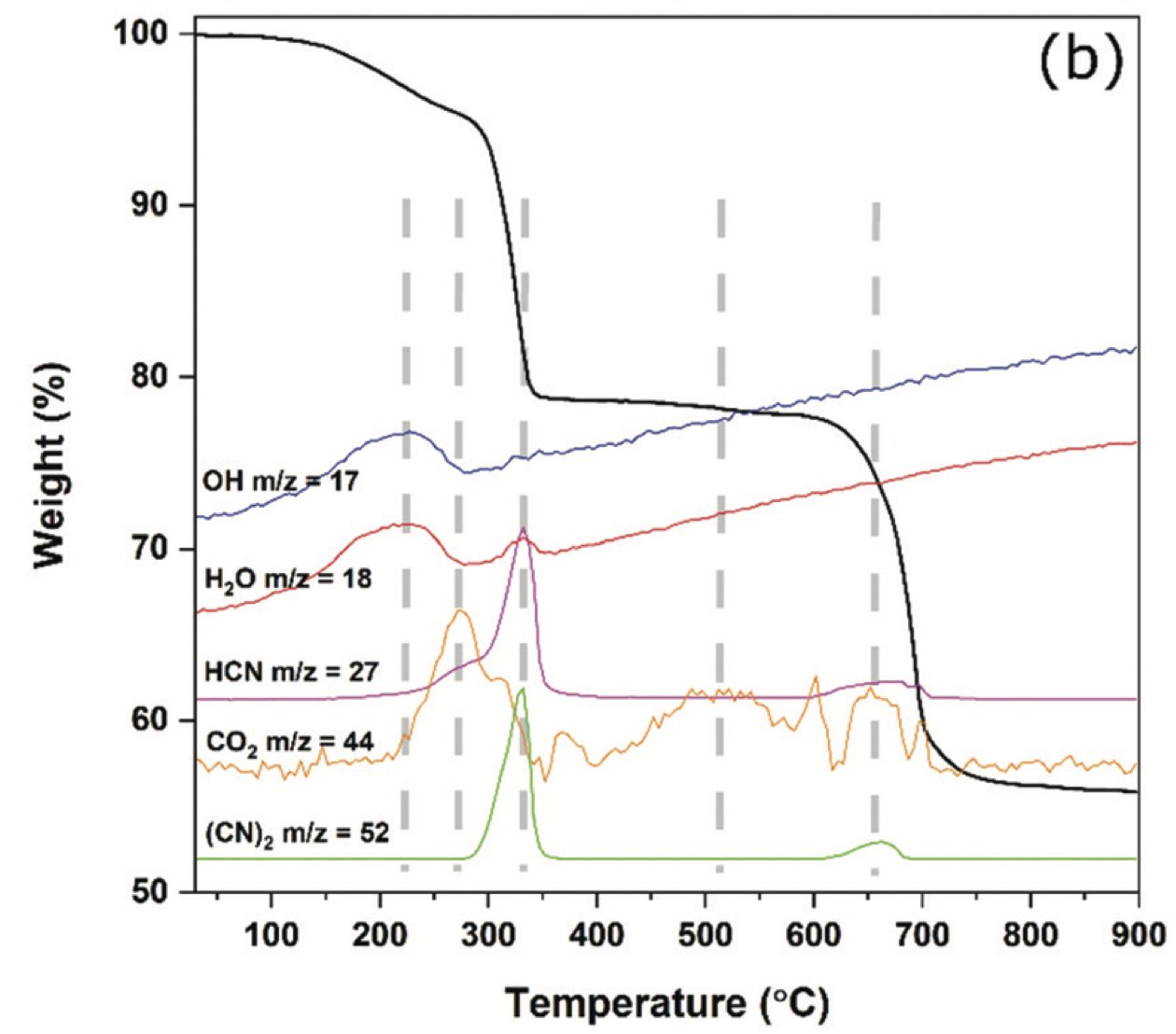
A more recent study has also looked at the stability of PBAs in contact with electrolyte and found similar gas evolution, but also an exothermic reaction with the electrolyte.
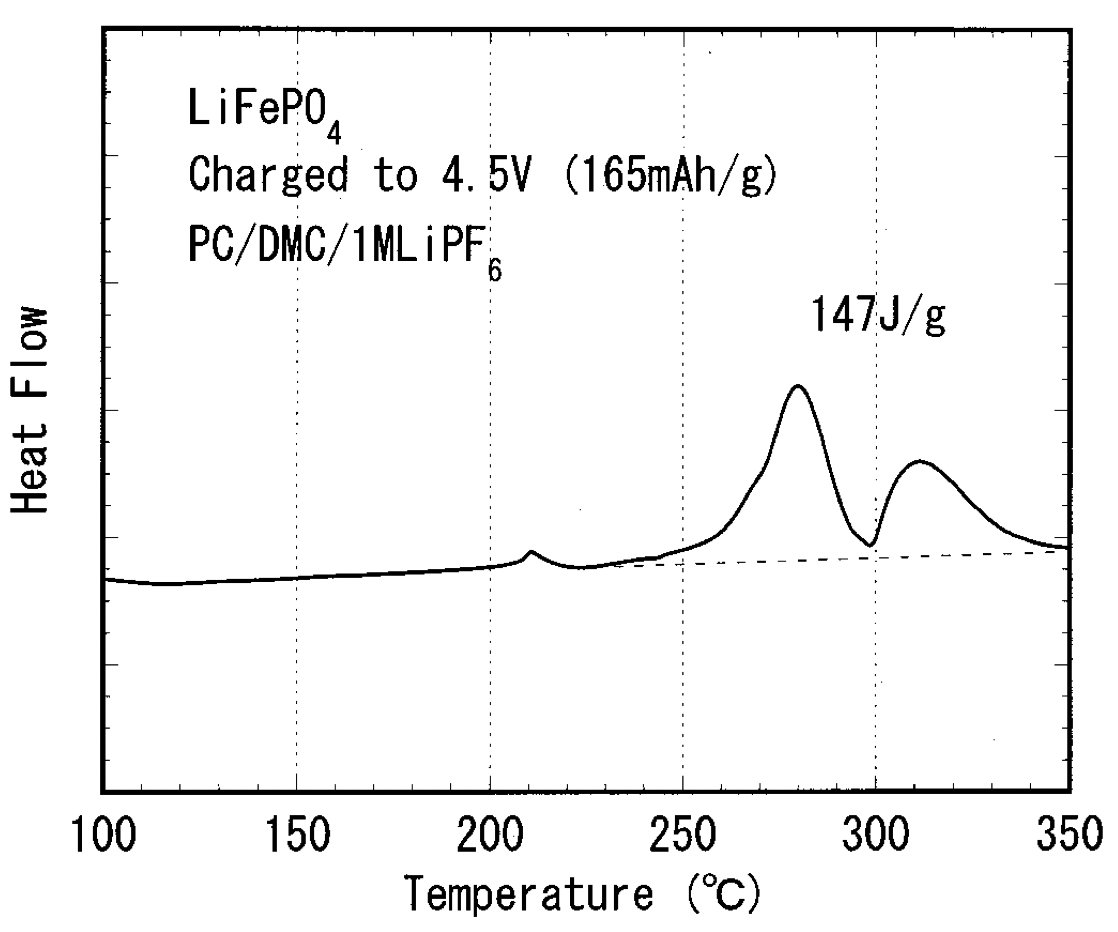
One thing that caught my eye was the magnitude of the heat release from DSC experiments. For a similar Fe-based PBA, the heat release was measured at 539 J/g. Looking elsewhere in the literature you find similar numbers for NMC111 and ~3x what has been measured for LFP…
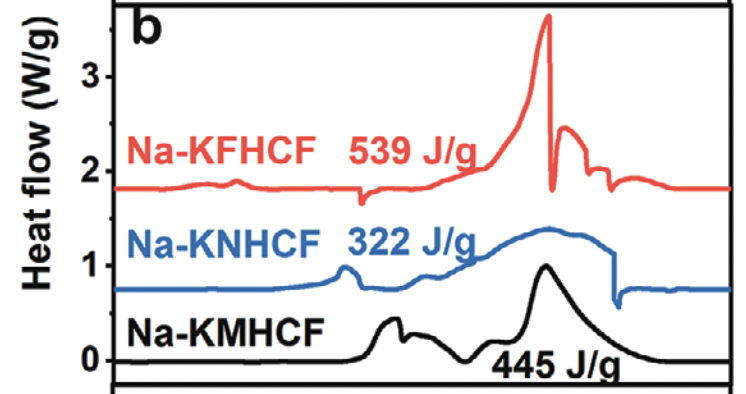
It’s also more than what the same group measured for NaCoO2 in another paper from last year (which interestingly was found to be more frisky when discharged).
Anyway, I think the comparison with LFP is most interesting because this is where the Na-ion cells are best positioned to compete, on cost, sustainability, energy density, etc. Does this mean the PBA Na-ion cells will be more unsafe than LFP?
I’d say not necessarily, there are too many unknowns at the moment. But maybe we can hypothesise a bit. LFP and PBA have similar specific capacities (mAh/g), so two cells of the same capacity will have similar amounts of cathode.
If the heat release is as indicated earlier, the Na-ion cell could be expected to have a more energetic thermal runaway, with a similar-ish onset temperature.
But, the Na-ion cell will have lower Wh/L, so more ‘thermal ballast’, and other differences (anode, SEI, electrolyte stability, etc) will affect the process as well.
This is also not to suggest either that the propagation can’t be controlled on system level, especially not now that CATL are presenting high-Ni CTP batteries with very high system efficiencies.
However, experience tells us that even packs where cell-to-cell propagation is supposedly eliminated can still become large fires in the worst cases, so I don’t believe the possibility can ever be ruled out while you have a combustible electrolyte and potential exotherms. So it is hard to make too many predictions, especially since I am not aware that there are any (public) results of safety testing on any large PBA cell yet.
One thing I would definitely conclude however is that there’s currently no basis on which to declare PBA Na-ion as safe, or at no risk of thermal runaway. In fact the reports of very large HCN and (CN)2 release I think need much more careful consideration.
The immediate danger to life and health (IDLH) level for HCN is set by NIOSH as 50 ppm. This is twenty-four times lower than the value for carbon monoxide (CO), the main toxic gas concern for conventional LIBs.
What this means is that even if you have 5% of the CN content of only one relatively large (say, 200 Ah) cell released as HCN, it needs to be diluted into >600 m3 to be below 50 ppm.
This would be pretty bad in an enclosed space, e.g. a parking garage or tunnel - and most people cannot smell HCN for genetic reasons.
Anyway, tl,dr: Recent research suggests PBA Na-ion cells not only undergo thermal runaway, but potentially quite energetically - & come with a unique toxic hazard which doesn’t seem to have gotten very much attention yet despite upcoming market introduction. Needs more study!
addendum: I was looking for info from CATL on the safety of their cells but couldn’t find more than a statement that they pass the relevant safety tests, which is not particularly informative. Would be very interested to see more detailed data if ever available.