Modelling LFP-Li cells
So, @DennisKopljar wanted to know what could in principle be achieved with LFP vs Li metal, and now we have another code walk through, part 2!
Tl;dr - LFP/solid state/Li will could get you roughly the same as NMC811/Gr, perhaps better. Let’s go:
I’m considering a seemingly sensible NMC811/Gr pouch cell as my baseline case (64 Ah, 257 Wh/kg, 634 Wh/L, 30 x 10 x ~1 cm)
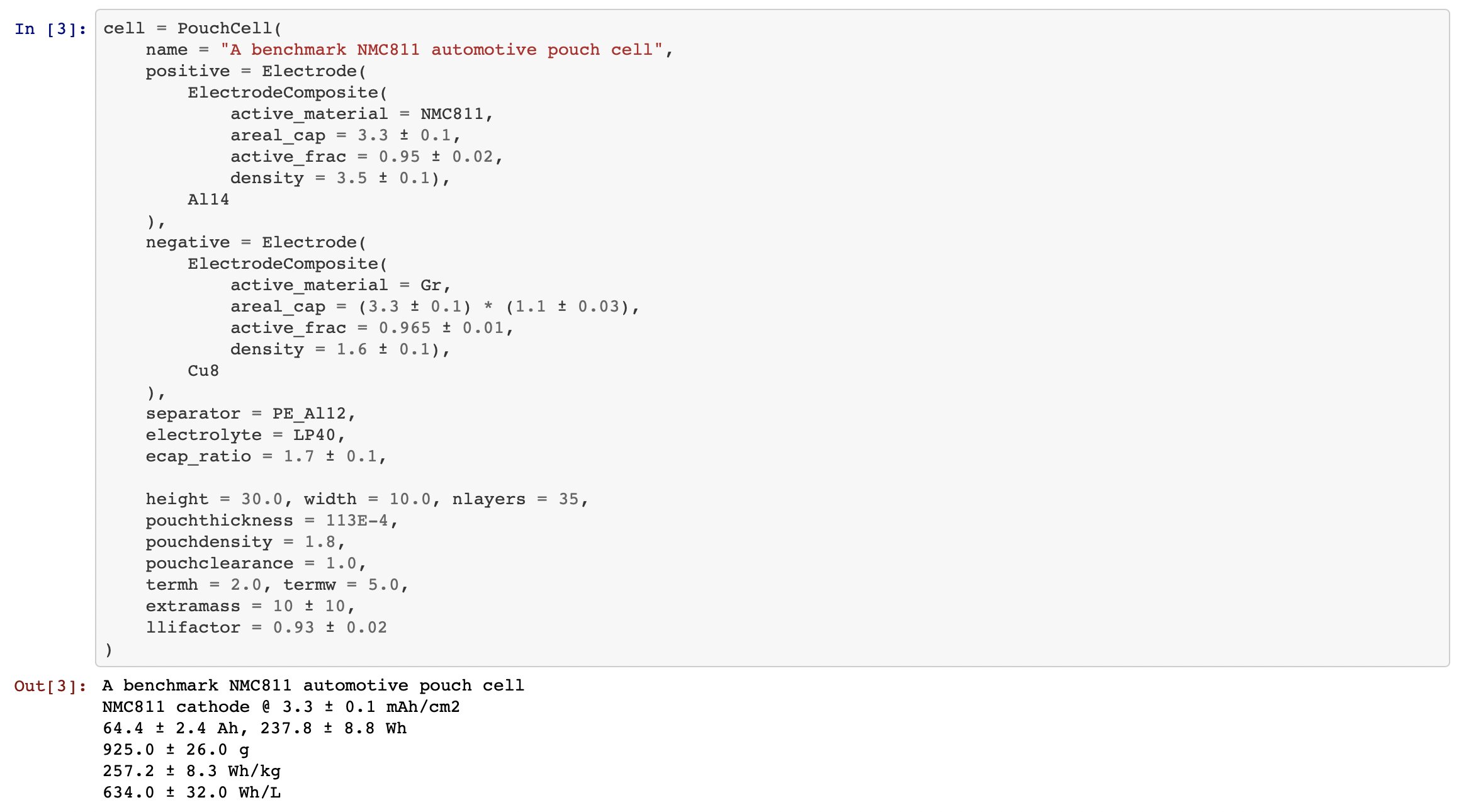
If all else stays the same and we assume LFP electrodes with same areal capacity, porosity etc… we get ~208 Wh/kg and ~451 Wh/L for the LFP, which seems on par with the SOA being developed in China. So far so good.
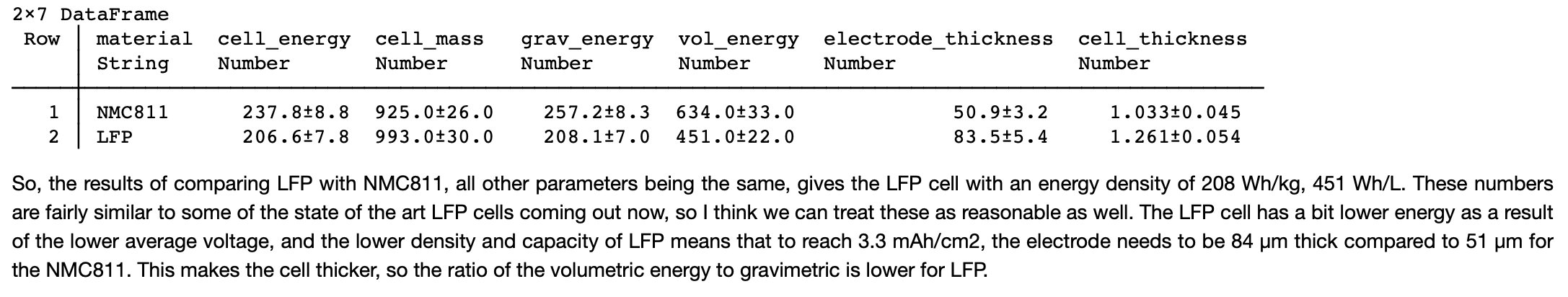
Case 1: Li metal. Assuming 50 µm Li metal foil, liquid electrolyte. This would improve energy density a fair bit, gravimetric more so than volumetric. LFP version is a bit higher Wh/kg than NMC811/Gr but lower volumetric.
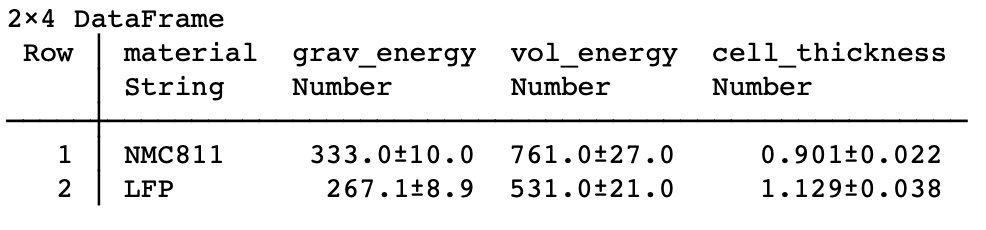
Case 2: Solid state, assuming an oxide (LLZO) at 20 µm thickness, keeping the same 50 µm Li foil. Decreases energy density compared to Case 1 because of heavier (and thicker) solid electrolyte. But still better than graphite anode.
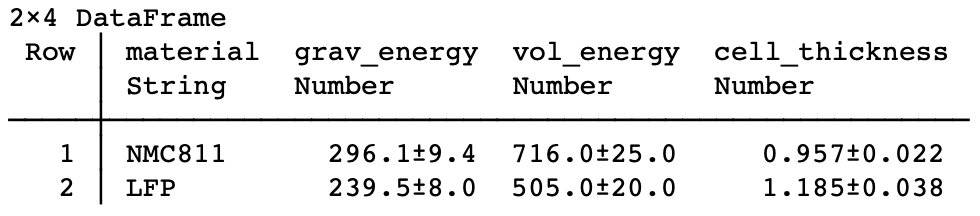
| Case 3: Anode-free, solid oxide separator (possibly in the style of a certain California-based solid state company): improved energy density, especially volumetric. Remarkably, LFP | LLZO | Li is almost the same on both grav and vol energy compared to NMC811/Gr! |
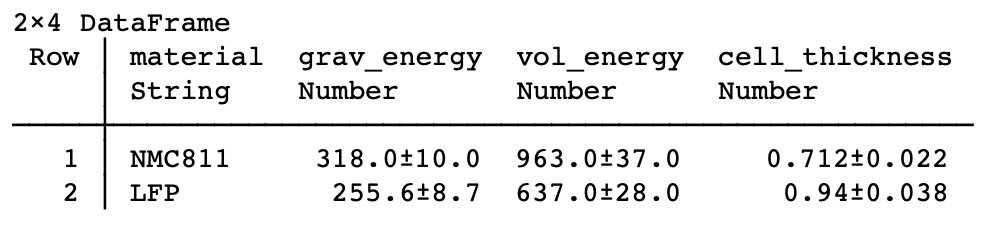
| Case 4: Sulfide electrolyte (possibly in the style of a certain Colorado-based solid state company): same volumetric (assuming same thicknesses), higher Wh/kg because of less dense electrolyte. LFP | sulfide | Li exceeds NMC811/Gr. |
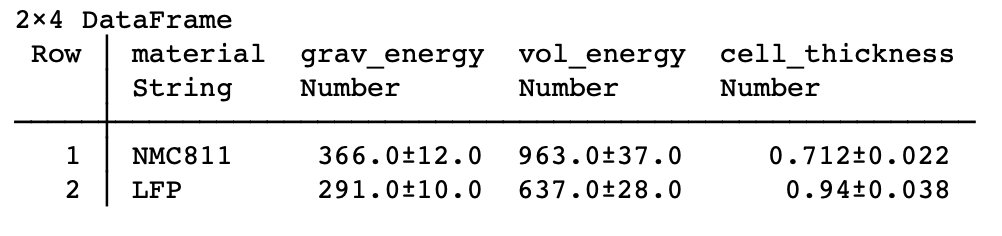
Summary: LFP vs Li solid state could match or better NMC811/Gr on energy density, so easy to see why it’s attracting interest. Could cut more costs, surely would be safer!
Be sure to check out the notebook linked above to see how it’s all done.
P.S. This is to say nothing about power, cycle life, or any of that stuff… that’s more complicated!