Electrolyte rate limitations
A paper which I would recommend reading. But it slightly annoys me, even though I agree with it. One might get the impression electrolyte limitations in battery electrodes have not been well studied, but we have known this for a long time - it just gets ignored.
While I was a 1st year PhD student, an MIT paper on an LFP coating was widely publicised on the basis that it would enable batteries that could charge in seconds, and it caused quite the controversy in the field.
My supervisor, John Owen, saw that this was misleading because of the remaining limitation from the electrolyte in the thick electrodes which are needed for practical batteries, and later published this paper, partly in response.
My experience over the years tells me that many materials scientists assume the limitation is always in the solid phase because of lower diffusion coefficients. Which is true, but electrolytes have longer diffusion lengths, much lower concentration, and mobile anions.
There’s actually a lot of work out there acknowledging this limitation and trying to overcome it. I have mentioned some of this before on Twitter, last year.
Part of the ignorance lies, I think, in choice of data presentation. In that same presentation, I had this slide. Look at the bottom right: capacity vs current density curves overlap almost perfectly for a large range in electrode thicknesses.
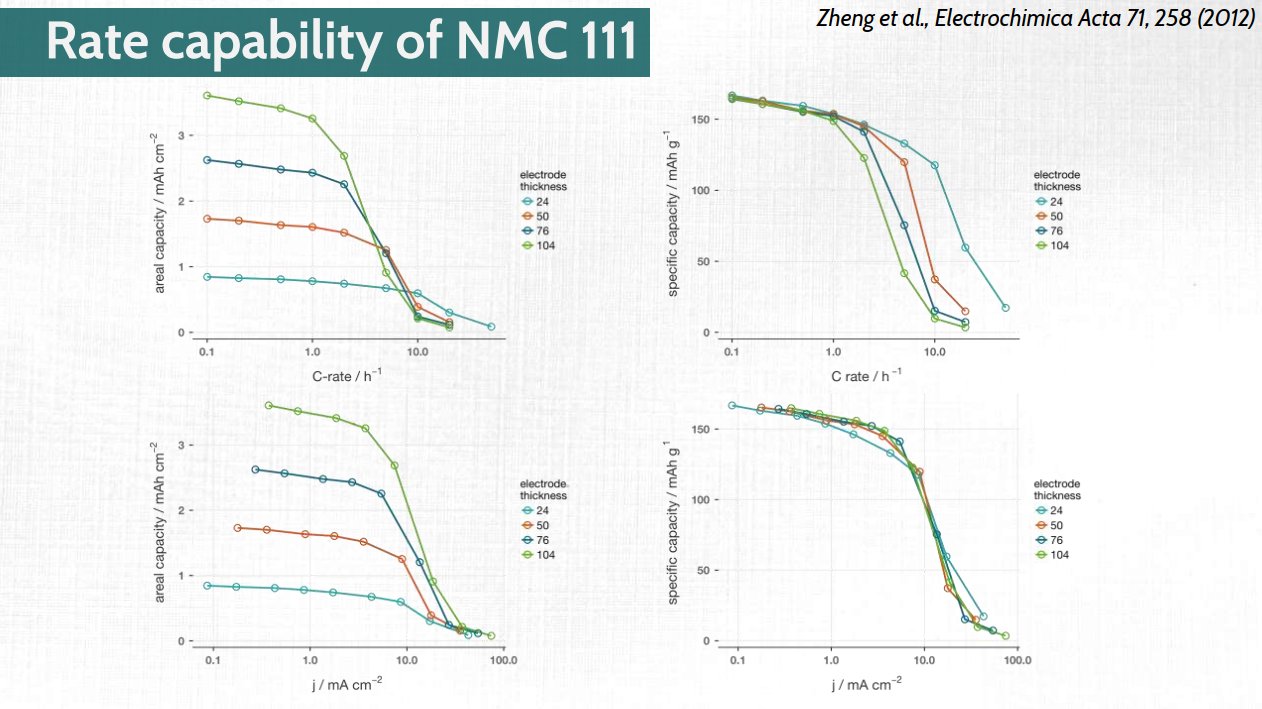
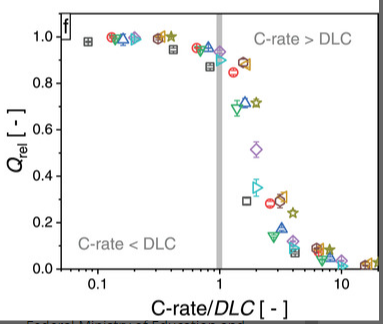
In Heubner et al (the first paper I linked) they introduce the concept of “diffusion-limited C-rate (DLC)”, which is areal current density divided by areal capacity. Notice the similarity?
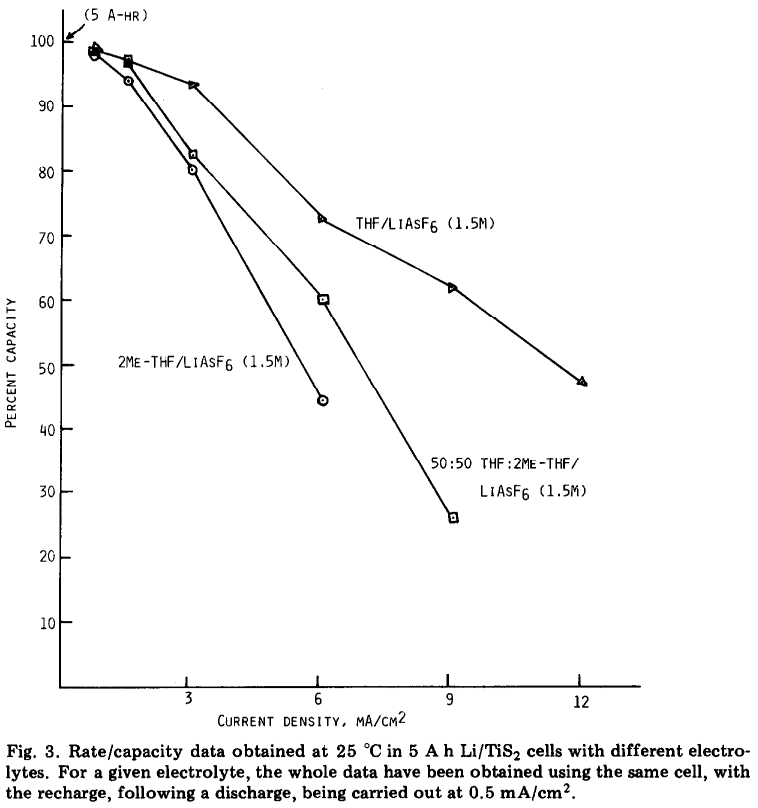
Interestingly, if you look at battery papers which were published in, say, the 1980s, rate capability plots which have mA/cm2 are rather common. From a 1985 review by K.M. Abraham:
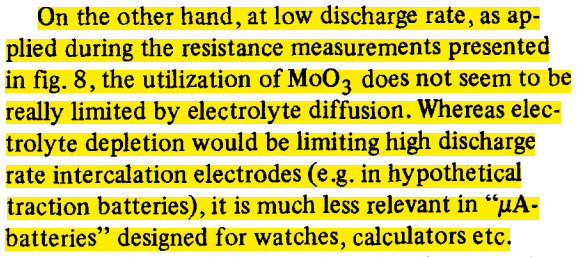
Electrolyte limitations were at least acknowledged to likely be limiting for high rates in real cases as well - did not take me long at all to find this example from 1983:
My hunch (and it’s just a hunch) is that the convenience of modern software for producing plots such as the classic “rate capability” plot (below) and an unhealthy obsession with “C-rate” have blinded a generation of researchers to important knowledge we already had.
I understand the pressure to show better and better performance from new materials in published work, but what how “impactful” is it if you can make an electrode that discharges at 100 C if it only does so for 0.1 mAh/cm2 electrodes?
I’ve long urged (students especially) to give careful thought about how performance should be understood in the lab context but also on how data should be presented. If you do things just because others do it that way, what happens when the “wrong” way becomes the standard?
